
| MadSci Network: Biochemistry |
Hi Crystal,
Thanks for submitting your question to the MadSci Network. As you requested, I'll give you a short answer, and a more detailed answer to your question.
Short answer: Xylan is a polysaccharide made from xylose monomers. Another way to say this is to use the analogy, xylan is to xylose as cellulose is to glucose. Just as a cellulose molecule is a string of many glucose molecules, so a xylan molecule is a long string of xylose molecules.
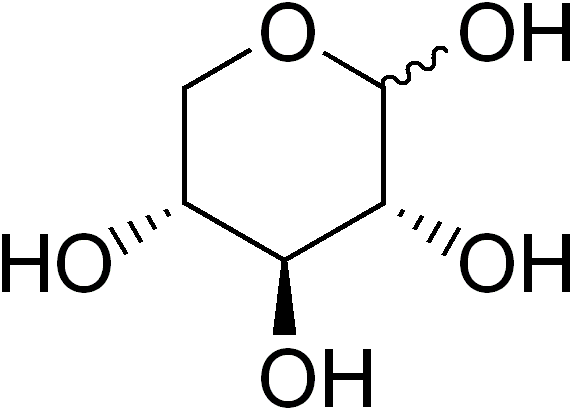
Long answer: Xylose is a sugar, chemically similar to glucose. Where glucose is a sugar built with six carbons (and is therefore called a hexose), xylose is a sugar built with only five carbons, and is a pentose. The structures of these two sugars are shown below.

| 
|
| Glucose | Xylose |
Sugars are characterized by many hydroxyl groups, and these allow them to be linked together into polymers -- long strings -- through enzymatic dehydration synthesis. Note that I put the word enzymatic in bold to make it clear that I am talking about a chemical reaction, not simply drying something out. In enzymatic dehydration of xylose to xylan, a hydrogen atom is removed from one xylose monomer, and one hydroxyl group is removed from the second sugar monomer, as described below (although the figure below is for the dehydration of amino acids), forming water and a new xylose dimer or xylobiose.

Finally, xylan polymers are characterized by beta 1-4 linkages between xylose monomers.
So, it sounds like your employer may be using the term dehydration incorrectly. If you take a xylose solution and heat it up slowly, evaporating away all of the water, you will not generate xylan. Similarly, if you soak xylan in water, you will not generate xylose. Specific enzymes (known as xylanases) are required to catalyse the hydrolysis reaction that adds water across the beta 1-4 bonds between xylose monomers in xylan, breaking them apart. Similarly, enzymes are responsible for the synthesis of new xylan molecules from xylose.
Here are some references so that you an verify this information:
The Reference.com entry on Xylanase,
The Dictionary.com entries on xylan, and xylose.
And here are some papers that present past and current research into this topic:
Konieczna-Molenda A, Lai VM, Fiedorowicz M, Khachatryan G, Tomasik P.
Polarized-Light-Stimulated Enzymatic Hydrolysis of Xylan.
Biotechnol Prog. 2008 Mar 4 [Epub ahead of print]
Akpinar O, Ak O, Kavas A, Bakir U, Yilmaz L.
Enzymatic production of xylooligosaccharides from cotton stalks.
J Agric Food Chem. 2007 Jul 11;55(14):5544-51.
Bailey Rw, Gaillard Bd.
Carbohydrases Of The Rumen Ciliate Epidinium Ecaudatum (Crawley). Hydrolysis Of Plant Hemicellulose
Fractions And Beta-Linked Glucose Polymers.
Biochem J. 1965 Jun;95:758-66.
Try the links in the MadSci Library for more information on Biochemistry.