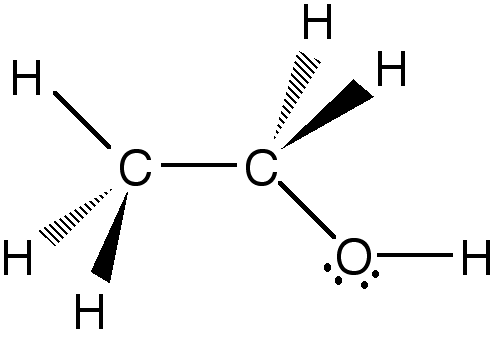
The filled triangles represent bonds coming out of the plane and the hatched triangles represent bonds going into the plane. The other two sp3 hybrid orbitals of oxygen are filled by paired electrons, shown as dots.
| MadSci Network: Chemistry |
Ethanol, or ethyl alcohol, has the stoichiometric formula,
C2H6O, but it is usually written as either
C2H5OH or CH3CH2OH in order to
distinguish it from dimethyl ether. Ethanol is composed of two carbon atoms
covalently attached by an sp3-sp3 molecular orbital, one of which is
covalently bound to oxygen by another sp3-sp3 molecular orbital. The
remaining free sp3 hybrid orbitals of the carbons and oxygen are bound to
hydrogens via sp3-s covalent bonds:

The filled triangles represent bonds coming out of the plane and the hatched
triangles represent bonds going into the plane. The other two sp3 hybrid
orbitals of oxygen are filled by paired electrons, shown as dots.
For more information on ethanol's structure and chemistry, try one of the following:
Try the links in the MadSci Library for more information on Chemistry.