Date: Sat Jun 11 21:22:41 2005
Posted By: John Link, Senior Staff Physicist
Area of science: Physics
ID: 1118450766.Ph
Message:
Be sure to visit our archives using
our search engine to
search on "radioactive decay". In many of the previous answers you will
find equations for determining the "decay constant" and halflife of
radioactive particles. The decay constant, L, is
L = ln(2) / T1/2
where T1/2 is the halflife and "ln" is the natural logarithm,
with ln(2) being about 0.693. After the decay constant is found, the
equation for the number of particles, N, after a certain time, T, is
N = No e-LT
where No is the number of particles at time T=0.
Here is a picture of an Excel chart containing the data you sent, along
with an exponential trendline that is the best fit to your data:
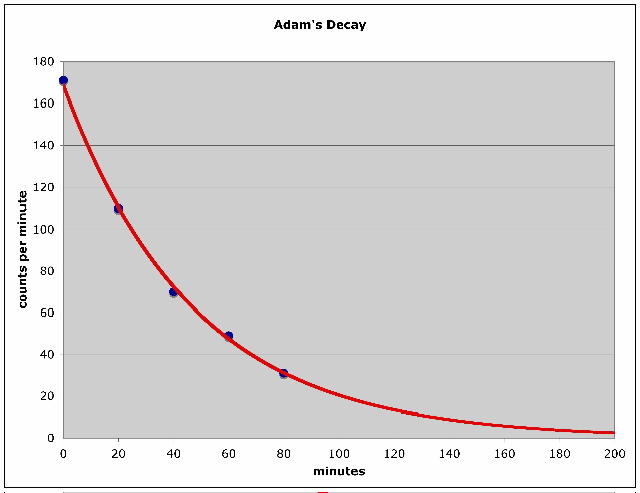
From these data Excel computes a
decay constant of 0.0211 per minute and an No equal to 168.7,
from which the halflife can be computed as 32.9 minutes. Let's calculate
the number of particles at T=1440 minutes (24 hours):
N = 168.7 e-0.0211*1440 = 1.1E-11
which is reasonably close to your calculation of 1.6E-11. But things are
not so clean when we are dealing with small numbers of particles, and,
indeed, is there even such a thing as a fraction of a particle? The answer
to that question is "no" and accounts for the fact that your teacher is
correct in saying there will be zero particles after 24 hours. Starting at
171 particles there is only 1 particle left after about 7 halflives. 24
hours is 43.8 halflives, so the one remaining particle would have to
not decay after about 36 or so halflives! While there is a finite
probability of that happening, the probability is extremely small, so it is
safe to say that at the end of the 24 hours the number of particles left
will be zero.
The probability of any one particle not decaying after a time of one
halflife is 50% (0.5). You can verify that by thinking of starting with,
say, 4 particles. After one halflife, on average, 2 will have decayed. So
after one halflife only 50% of the particles decayed. When we get down to
only 1 particle left there's a 50% chance (0.5) that it will not decay
before one halflife is ended. If it does not decay after that time,
there's still only a 50% chance that it will not decay after an additional
halflife time, so that after 2 halflives there is only a 25% chance (0.5 *
0.5) that it will not have decayed. In your problem the one particle has
about 36 halflives to get through without decaying, which means that the
chance of that happening is
0.536 = 1.5E-11 = 1.5E-9%. That is
a small enough probability to accept the fact that that single particle
will not be there at the end of the 24 hours.
John Link, MadSci Physicist
Current Queue |
Current Queue for Physics |
Physics archives
Try the links in the MadSci Library for more information on Physics.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@madsci.org
© 1995-2005. All rights reserved.
