| MadSci Network: Biochemistry |
Hi K,
There are many rules regarding solubility: miscibility, temperature, pressure, ionic, polarity and more are all factors.
However, truth be known, almost every chemical has a partial solubility partition [δS(p)] in any solvent. A high partition and the substance dissolves very easily, the lower the partition, and it dissolves less well, until it is almost insoluble.
In other words, a certain amount of cellulose will dissolve in water, but it will be very little.
This can be easily confirmed by vortex mixing of cellulose in H2O followed with turbidity measurement. Use the proper controls and a Spect 20 set to the blue end of the spectrum -- 400 to about 470 nm.
There is a rule of thumb regarding solubility among chemists -- less than 3 percent of solute in the solvent and it is reasonably considered insoluble in that solvent. Unfortunately, I could not find a reliable reference for that.
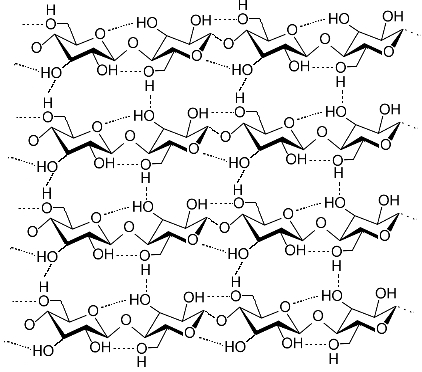
Below is the structure of cellulose. One can see readily that it is so sterically hindered, so dense, that solubility in water would be difficult.

But cellulose does have a δS(p) according to:
a. length of chain and
b. density between chains.
As a biochemist, I must add to this discussion that those factors and partitions are very important to the human diet and health.
If the chains are separated and allowed to flow through the human digestive system [a high δS(p)], they are soluble fiber. They retain fluid and vitamins throughout the intestine. They contribute to soft stool.
If the chains are not separated, but macerated [a low δS(p)], they remain firm enough that when allowed to flow through the human digestive system, they are able to remove pre-cancerous colon polyps. These contribute to a more firm stool.
If the density is changed by being broken into very tiny small dense pieces or in long chains, then some portion is solvated.
Cellulose should be generally regarded as insoluble in water, but is certainly not perfectly insoluble.
Thank you for your interesting question!
Peter Hughes, Biochemist and Mad Scientist
http://www.chem.fsu.edu/chemlab/chm1046course/solubility.html
http://www.cmcsanhui.com/about_CMC_technical.html
http://eosweb.larc.nasa.gov/EDDOCS/Wavelengths_for_Colors.html
Try the links in the MadSci Library for more information on Biochemistry.