Fructose (C6H12O6)
Ribose (C5H10O5)

Hydroxymethylfurfural (C6H6O3)

Furfural (C5H4O2)
| MadSci Network: Biochemistry |
Hi.
I read in a text book about the furfural derivatives.
I searched about the meaning of furfural but I do not get it.
Why does the dehydration of hexoses give hydroxymethylfurfural and that of pentoses give furfural?
What is the difference between hydroxymethylfurfural and furfural?
It might help for you to take a look at some examples of these molecules.
| SIX CARBONS | FIVE CARBONS | |
| SUGARS |
Fructose (C6H12O6) |
Ribose (C5H10O5) |
| FURFURALS |
 Hydroxymethylfurfural (C6H6O3) |
 Furfural (C5H4O2) |
So, as you will note in the table above, hexoses are six-carbon sugars, and pentoses are five-carbon
sugars.
If
you count the number of carbons in hydroxymethylfurfural and furfural, you will see that they are
also six-carbon and five-carbon molecules, respectively.
The difference between each sugar and its
corresponding furfural is three water lost in the dehydration process.
I've drawn an example of this in the figure below, which is inspired by Scheme I in the paper by de Souza et al. 2012 a the bottom of this answer.
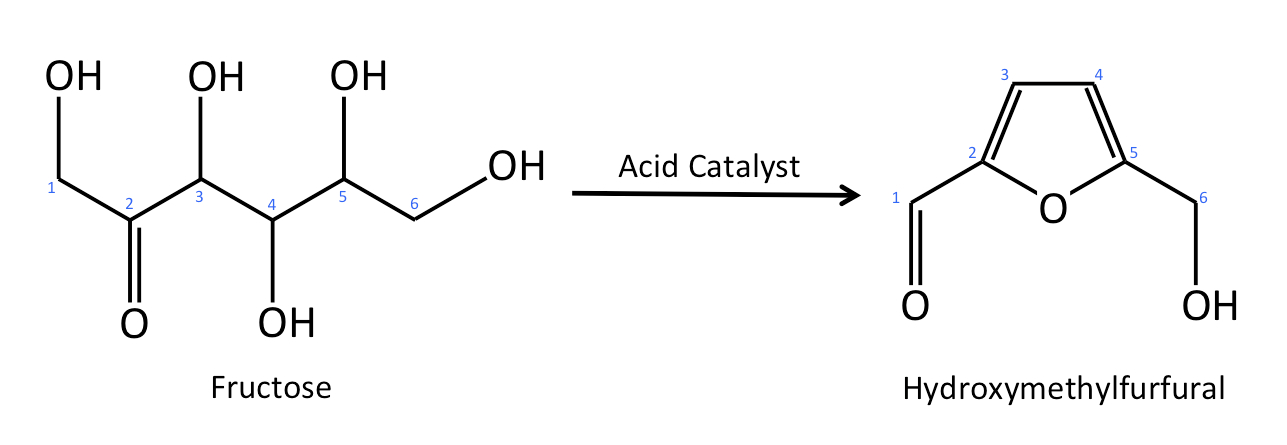
In this figure, fructose (shown in linear form) is dehydrated to hydroxymethylfurfural via an acid catalyst.
I have numbered the carbon atoms (from 1-6) in each molecule, and if you pay close attention, you can see
that the hydroxyl groups and hydrogens
associated with carbons 3, 4, and 5 in the fructose molecule have been removed (dehydrated); there
follows quite a lot of shifting around of electrons,
which results in the formation of the hydroxymethylfurfural molecule.
The same thing happens with the dehydration of pentose to furfural. I think that if you draw out the ribose and furfural structures, you will be able to observe the same phenomenon.
Keep asking questions!
Reference:
de Souza R.L., Yu H., Rataboul F., Essayem N.
5-Hydroxymethylfurfural (5-HMF) Production from Hexoses: Limits of Heterogeneous Catalysis in
Hydrothermal Conditions and Potential of Concentrated Aqueous Organic Acids as Reactive Solvent
System
Challenges 2012, 3, 212-232. doi:10.3390/challe3020212
www.mdpi.com/2078-
1547/3/2/212/pdf
Try the links in the MadSci Library for more information on Biochemistry.