Re: What is a carbonyl group?
Area: Chemistry
Posted By: Dan Berger, Faculty Chemistry/Science, Bluffton College
Date: Fri Feb 7 13:21:06 1997
Message:
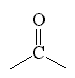 A carbonyl group is a carbon-oxygen double bond. Oxygen can have only two bonds, so nothing else can be bonded to oxygen, but carbon forms four bonds, so there are two "spaces" left over in which other atoms can be bonded to the carbon. A carbonyl group looks like the picture at the left.
A carbonyl group is a carbon-oxygen double bond. Oxygen can have only two bonds, so nothing else can be bonded to oxygen, but carbon forms four bonds, so there are two "spaces" left over in which other atoms can be bonded to the carbon. A carbonyl group looks like the picture at the left.
Examples of compounds with carbonyl groups are shown below, and include:
- Benzaldehyde, which gives almonds their odor;
- Methyl Ethyl Ketone, a strong, sweet-smelling compound that is sometimes used in paints;
- Acetic Acid, the active ingredient in vinegar; and
- Ethyl Acetate, another sweet-smelling compound which can be used as a flavoring. It is produced in small amounts by the yeast which makes bread rise.
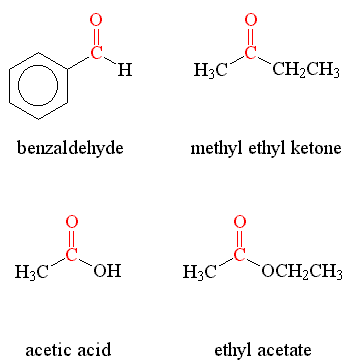 Notice that in the compounds shown above, the carbonyl groups have been shown in red.
Notice that in the compounds shown above, the carbonyl groups have been shown in red.
For more information about organic compounds and their structure, try Dave Woodcock's organic nomenclature tutorial.
DJB
Current Queue |
Current Queue for Chemistry |
Chemistry archives
Try the links in the MadSci Library for more information on Chemistry.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network
© 1997, Washington University Medical School
webadmin@www.madsci.org
 A carbonyl group is a carbon-oxygen double bond. Oxygen can have only two bonds, so nothing else can be bonded to oxygen, but carbon forms four bonds, so there are two "spaces" left over in which other atoms can be bonded to the carbon. A carbonyl group looks like the picture at the left.
A carbonyl group is a carbon-oxygen double bond. Oxygen can have only two bonds, so nothing else can be bonded to oxygen, but carbon forms four bonds, so there are two "spaces" left over in which other atoms can be bonded to the carbon. A carbonyl group looks like the picture at the left.
